Abstract
Introduction: Unfractionated heparin (UFH) is the first line anticoagulant for the management of medical indications. UFH complexes with antithrombin to produce strong inhibition of thrombin and factor Xa. The UFHs are standardized using USP compliant amidolytic anti-Xa and IIa methods in defined conditions. Clinically used UFH is solely sourced from porcine mucosal tissue. Because of the shortage of porcine tissue and the African Swine Fever, the supply chain of this anticoagulant is compromised. Thus, there is a need for resourcing of this anticoagulant. Bovine and ovine mucosal sources represent alternate material for production of UFH. Previous studies have shown that bovine and ovine UFH exhibit anticoagulant effects which can be standardized by using the USP method. Additionally, the standardized heparins from various sources can be blended and their potency can be adjusted to exhibit comparable effects as the single sourced UFH. The purpose of this study is to evaluate the pharmacologic profile of the blended heparin and compare these activities to that of the single sourced porcine, ovine and bovine heparins.
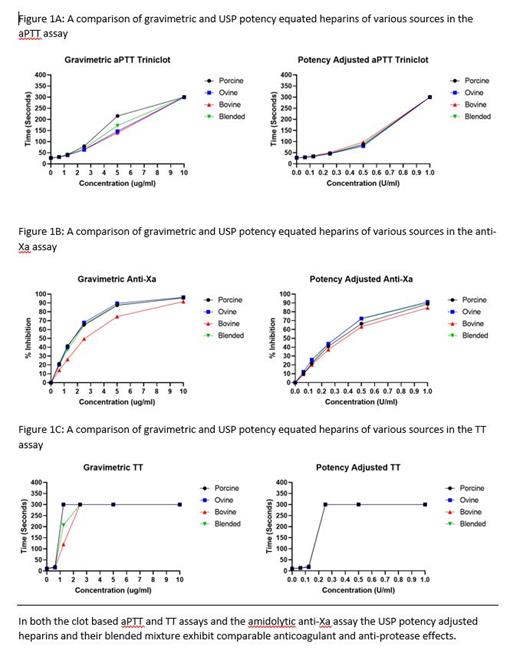
Methods: Two groups of heparins were evaluated in this study, porcine, ovine, bovine, and the blended heparin in gravimetric measurements (ug/ml) and these same four in potency adjusted measurements (U/ml). The pharmacologic profiles of the heparins in this study were investigated via global anticoagulant assays and anti-protease assays performed in plasma. Clot based assays such as the activated partial thromboplastin time (aPTT) and thrombin time (TT) were used to study the anticoagulant effects of the single source and blended heparins. The amidolytic anti-Xa and IIa assays were used to assess the inhibitory effects of these heparins on these proteases. USP compliant anti-Xa and IIa assays were used to determine potencies of the various heparins. Protamine sulfate (PS) neutralization studies were performed to evaluate the reversal of anticoagulant effects in each of the heparins.
Results: The aPTT assay showed that at final concentrations of 5 ug/ml and 2.5 ug/ml porcine heparin significantly (p < .01) prolonged the aPTT compared to ovine, bovine, and blended heparins. When studied with potency adjusted heparins, all heparins demonstrated comparable aPTT values at all concentrations (U/ml). The TT assay showed that porcine and ovine heparins prolonged the TT at 1.25 ug/ml compared to bovine and blended heparins. When studied with potency adjusted heparins, all heparins demonstrated comparable TT values at all concentrations (U/ml). The anti-Xa assay showed that at all final concentrations between 10 ug/ml and 0.625 ug/ml porcine, ovine, and blended heparins produced significantly (p <.001) stronger Xa inhibition than bovine heparin. When studied with potency adjusted heparins, all heparins demonstrated comparable anti-Xa inhibition at all concentrations (U/ml). The anti-IIa assay showed that at final concentrations 2.5 ug/ml, 1.25 ug/ml, and 0.625 ug/ml porcine and ovine heparins produced significantly (p < .05) stronger IIa inhibition than bovine heparin. When studied with potency adjusted heparins, all heparins demonstrated comparable anti-IIa inhibition at all concentrations (U/ml). The USP compliant anti-Xa assay with gravimetric heparins showed potencies of 201, 201, 150, and 184 U for porcine, ovine, bovine, and blended heparins respectively. The USP compliant anti-Xa assay with potency adjusted heparins showed comparable potencies for all four heparins. The USP compliant anti-IIa assay with gravimetric heparins showed potencies of 204, 196, 127, and 167 U for porcine, ovine, bovine, and blended heparins respectively. The USP compliant anti-IIa assay with potency adjusted heparins showed comparable potencies for all four heparins. The protamine sulfate neutralization studies demonstrated complete neutralization at all concentrations for all of the potency adjusted heparins in the aPTT, TT, anti-Xa, and anti-IIa assays.
Conclusion: These studies support the hypothesis that a blended heparin product from bovine, ovine, and porcine tissue, when standardized in USP unit-equivalent proportions, exhibits a comparable anticoagulant profile to the single species heparins. These findings suggest that there is a potential for development of blended heparin to stabilize supply chain of this important anticoagulant and warrant clinical validation.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal